DRY EYE IS A DISEASE
We provide a wide range of services to manage chronic and acute dry eye, a common medical condition, including the newest treatment for ocular surface disease: the use of an Amniotic Membrane Allograft called Cam360®.
DRY EYE CENTER OF EXCELLENCE
Ophthalmology Associates of Osborne offers many innovative ways to treat dry eye and tear film dysfunction. These solutions can improve your day to-day vision and relieve you of your symptoms. If your eyes are uncomfortable, dry, constantly tired feeling, red, or in pain, you may be experiencing a problem with your corneal health, eyelid glands, tear film evaporation, or tear production. Or a combination of these. Call us at
(412) 741-6776
to schedule a consultation for treatment options to feel and see more comfortably!
What is Dry Eye?
In a healthy eye, tear production and proper blinking are responsible for the perfectly smooth ocular surface which allows clear vision. They are important for keeping the eye moist and comfortable. Our tear film also protects the corneal nerves from being exposed and becoming inflamed and irritated. Corneal nerves are important for sending appropriate signals to the brain regarding comfort.
Sometimes, the eye may stop producing enough tears, or tears may evaporate too quickly. This can be due to underlying disease, excessive device use, medications, hormones, age, or environment. The eyelids have many tiny glands responsible for creating part of our tears and can also stop working as effectively with age, inflammation, and progressive blockage. Eye surgery can also impact tear function.
Therefore, this condition is often chronic and multi-factorial!!
There is no single 'cure' but fortunately, there are many ways to treat dry eye disease causes and symptoms. Our office offers new and innovative ways to help you manage this condition and provide you with longer lasting comfort and clearer vision.
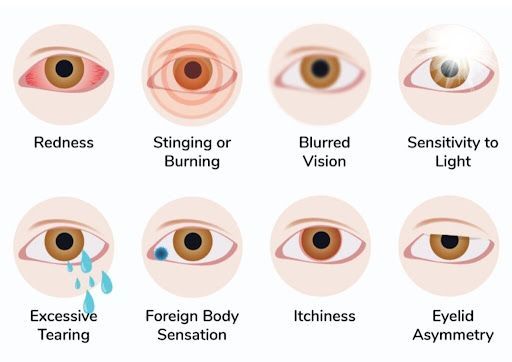
Typical symptoms of "dry eye" include: burning, stinging, itching, foreign body sensation, pain, fatigue, sensitivity to light, excess tearing, gritty sensation, and more. Some people may not have symptoms because the corneal nerves have become damaged or desensitized over time. Our doctors will look at your cornea and tear film during the exam, along with testing these for proper health and function.

COMMON SYMPTOMS OF DRY EYE
One method of treating dry eyes is with punctal plugs.
Punctal plugs are small, reversable, painless medical devices inserted into the tear duct in the office without anesthetic. They are placed in the tear duct to block them and keep them from draining liquid, which helps keep more tears on the surface of the eye for longer and improving comfort and vision. Keeping tears on the eye longer can help treat dry eyes. Punctal plug insertion can compliment other therapies, such as using artificial tears or prescription drops, more successful.

*NEW INNOVATIVE TREAMENT:
CAM 360 and PROKERA® For Dry Eye!
CAM 360 and PROKERA® are a type of amniotic membrane our office uses for ocular surface disease. They are devices used by eye doctors around the world for anti-inflammation, anti-scarring, and the promotion of healing damaged eye surfaces and nerve regeneration. CAM 360/Prokera are the only FDA-cleared cryopreserved amniotic membranes. They have specific advantages, but ultimately supports the corneal-healing process without harmful side effects or costs of drops/chemicals. Both are made from amniotic tissue which has natural anti-inflammatory and anti-scarring properties. It provides quick symptom relief and reduces inflammation associated with ocular surface disease.
It helps restore your cornea and return your eye to a normal, healthy state.
'Healing Vision, Restoring Life'
Bio-Tissue created CAM360 and Prokera to leverage the potent healing properties of amniotic tissue that is responsible for regeneration. It is cryopreserved to retain key benefits of this tissue that include suppressing ocular surface inflammation, decreasing corneal scarring and angiogenesis.
What advantage does cryopreserved amniotic tissue have over dehydrated amniotic tissue?
Heat dehydrated amniotic membrane grafts are processed in a way that destroys crucial biologic components, increasing the risks for repeated epithelial defects or erosions, chronic inflammation, scarring, vision loss, or pain.
What is amniotic membrane tissue?
An amniotic membrane has biological properties that aid in tissue repair. These tissues have natural therapeutic actions that help damaged eye surfaces heal. Eyes treated with this technique generally improve significantly and have less scarring and inflammation. The amniotic membrane in very thin, typically less than 50 microns, and generally comfortable.
Amniotic membranes are harvested and must be in immaculate condition and taken without complications. The process of harvesting and processing these tissues is tightly controlled under heavy restrictions.
What does CAM 360/PROKERA treat?
These medical devices are used by eye doctors to treat ocular surface diseases such as keratitis, corneal scars, chemical burns, corneal defects, partial limbal stem cell deficiency, Simplex keratitis and many other ocular surface diseases. They are provided by a tissue bank regulated by the FDA. The tissue has passed multiple severe quality control tests before being provided to your doctor. Ask your doctor if you are concerned about the risks involved with using human tissue. Here are some of many conditions CAM360/Prokera can help with:
- Dry Eye Syndrome, Exposure Keratopathy
- Filamentary Keratitis
- Infectious Keratitis
- Neurotrophic Persistent Corneal Epithelial Defect
- Recurrent Corneal Erosion
- Corneal Ulcers
- Pre-Cataract Optimization
- Chemical burns
- Post-DSEK for Bullous Keratopathy
- Salzmann’s Nodular Degeneration
Are Amniotic membranes safe?
CAM360/Prokera are safe treatments provided by an FDA regulated tissue bank. The tissue is donated from consenting, healthy mothers who have had an uncomplicated C-section. The donor and tissue have passed numerous quality control tests including social habits, physical and medical screening before it is provided to your doctor.
What should you expect?
CAM360/Prokera are similar to a large contact lens. You may experience an awareness while inserted, but they are not normally painful and generally comfortable. The amount of time the Prokera is inserted in your eye depends on the level of severity of corneal disease, but typically is from 2-5 days.
Special instructions for amniotic membrane use:
- Avoid rubbing your eyes, forceful blinking, or moving the membrane with your fingers
- Do not remove the membrane without consulting your eye doctor first
- Do not swim or soak your eye with water
- Shower only when the eye is tightly closed
- Do not drive or operate heavy machinery or perform functions that require unobstructed vision or good depth perception
- Use eye drops and other medications as prescribed by your eye doctor
Contact your eye doctor right away if you are uncomfortable or have any other problems with either device, such as swelling, redness, or discharge.
Proper Management is Vital for Optimal Vision
If you are concerned that you may be suffering from dry eye, call Ophthalmology Associates of Osborne at (412) 741-6776 today to schedule a consultation.
